https://doi.org/10.5281/zenodo.17594186
In ordinary solid–state physics, a temperature gradient across a conductor can drive an electric field even when no net current flows. In linear response, this is written as the familiar thermoelectric or Seebeck law
where is the Seebeck coefficient (thermopower). In Quantum Traction Theory (QTT), this macroscopic law is not just a phenomenological fit. It emerges from:
- the equilibrium of a capacity chemical potential,
- the fact that charge and heat are different dials of the same capacity ledger,
- and Planck–scale capacity bounds on electric fields and temperature gradients in each world–cell.
To avoid confusion with the QTT Absolute Clock , we denote thermodynamic temperature by
. The classical law becomes
with the thermoelectric coefficient.
1. Classical Seebeck effect from electrochemical equilibrium
Consider charge carriers of charge and number density
in a conductor. Let
be the chemical potential per carrier and
the electric potential. The electrochemical potential is
In static equilibrium with no net particle current, must be spatially constant:
Assuming a uniform carrier density (no accumulation), , we have
The equilibrium condition becomes
Using gives
This is usually rewritten in the form
This boxed equation is the standard microscopic definition of the Seebeck coefficient: the thermopower is given by the temperature derivative of the chemical potential per carrier, divided by the carrier charge.
2. QTT reinterpretation: chemical potential as capacity potential
QTT treats the chemical potential as a capacity potential. Each carrier corresponds to a dial configuration on a Planck–scale world–cell and carries:
- a U(1) dial charge
with the fundamental charge quantum; a thermal capacity per carrier
, such that
a capacity chemical potential , the tick–energy cost to add one more carrier to the world–cell ensemble.
QTT identifies
Then the Seebeck coefficient becomes
So in QTT the thermoelectric coefficient is explicitly:
- “change of capacity energy per carrier per unit
,”
- divided by the dial charge per carrier
.
In simple models, per carrier, so
. QTT interprets
as the natural ratio of a thermal capacity quantum to a dial charge quantum.
3. World–cell capacity bounds on 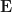 and
and 
QTT also imposes finite capacity per world–cell, which bounds both the electric field and the temperature gradient.
The electromagnetic energy density in the lab is . The QTT space quantum volume is
and the universal tick energy is
Requiring that a single space quantum never stores more than one tick energy gives
Similarly, if a temperature gradient across one cell of size would change the thermal capacity by more than the available per–cell capacity, it is not allowed. This yields a material–dependent upper bound
Together with , these bounds imply
so for a given material (fixed ) the allowed temperature gradient is limited by world–cell capacity.
4. QTT thermoelectric law (all boxed relations)
Collecting everything, the QTT version of the thermoelectric law is:
This boxed law contains all of the QTT thermoelectric structure:
- The field–gradient relation
.
- The microscopic definition of
as a capacity chemical–potential derivative per dial charge.
- Planck–regulated bounds on both
and
arising from finite electromagnetic and thermal capacity per world–cell.
5. What thermopower means in QTT
From the QTT viewpoint, the Seebeck effect is no longer just “hot carriers diffuse, so an electric field appears.” Instead:
- Heat and charge are different dials of the same capacity carried by world–cell configurations.
- A temperature gradient tilts the thermal dial, changing the capacity chemical potential
.
- The system restores equilibrium of electro–capacity by creating an electric potential gradient, i.e. an
field.
- All of this happens under strict Planck–scale capacity bounds on energy per cell and thermal gradients.
The classical law is still correct, but QTT exposes its deeper structure:
thermopower is the ratio of capacity energy per carrier to dial charge, and the resulting thermoelectric field is the Reality–Dimension–regulated response of a finite–capacity world–cell lattice.